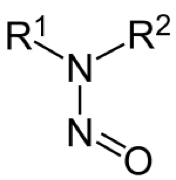
Nitrosamine, formally known as N-nitrosoamine, is a class of organic molecules with the above core structure. These type of chemicals are believed carcinogenic due to their potential as alylating reagents inside cells, which will cause cancer. These chemicals become huge concern in recent years due to FDA announced several drugs have been contaminated with nitrosoamine. However, these type of chemicals are widespread through the environment, in air, water and food.
In the past three years, FDA has reported several detection of high level of nitrosamine in marketed drugs. One more drug was added to the list in2022. Here ChemRealm.com listed the drugs are recalled due to nitrosamine contamination. with
| drug name | Time and Key action | Nitrosoamine name/analysis mehtod |
| Zantac | Discovered in Sep. 2019, Removed all ranitidine product from market in Apr. 2020 | NDMA: LC-HRMS and LC-MS/MS. Above the FDA recommended Daily intake level of 90 ng. |
| Angiotensin II Receptor Blocker (Valsartan Losartan Irbesartan Azilsartan Olmesartan Eprosartan Candesartan Telmisartan) | First recall in Jul. 2018 Most recent update: Nov. 2019 | NDMA NDEA, NDIPA NEIPA detection method by LC-MS/MS, GC-MS etc. |
| Metformin | First discovery: Dec. 2019 | NDMA: LC-HRMS |
| Rifampin and Rifapentine | First notice: Aug. 2020 | MNP, CPNP: LC-ESI-HRMS method, No drugs allowed on market if higher than 20 ppm of nitrosoamine |
| Varenicline(Chantix) | First Alert: Jul. 2021 | N-nitroso-varenicline: LC-ESI-HRMS method |
| Sitagliptin | Aug. 2022 | NTTP |
