What is Suzetrigine
Suzetrigine is the generic drug name for brand drug, Journavx. In Jan. 2025, FDA approved Vertec Pharmaceuticals to market the Journavx, a 50 mg tablet to treat short-term (acute) moderate to severe pain. As an alternative to opioids, it is the first pain medication approved by the Food and Drug Administration in two decades.
It was named as VX-548 during the developmen. Its molecular structure is as below:
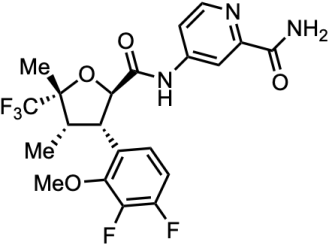
How is the Suzetrigine manufactured
In 2021, Vertec patented the molecule structure(WO2021113627A1) and outlined first generation synthesis route of the API, below. In this route, the API is synthesized non-enantioselectively, which at the end, requires chiral separation by SFC.
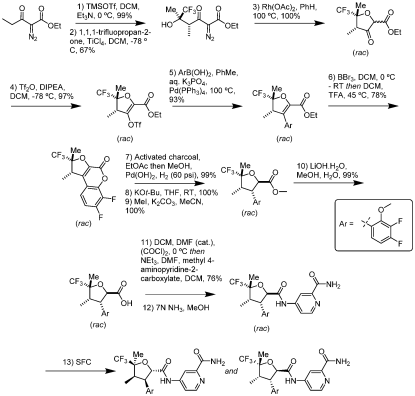
In 2022, a process for manufaturing thedrug was publised(WO2022256660A1) by Vertex with imporved efficiency and chiral resolution with quinine.
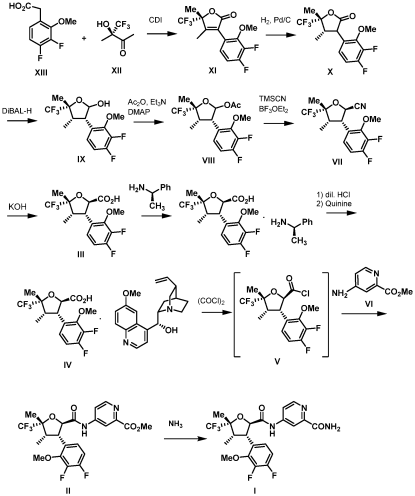
How does Suzetrigine work
Journavx’s mechanism of action is by selectively blocking NaV1.8, a sodium channel on pain-sensing nerve cells (neurons), this inhibits pain signals going to the spinal cord and brain. It relieves pain by blocking pain-sensing nerves around the body from transmitting pain messages to the spinal cord and brain. Since it does not affect the brain, is expected to have no addictive potential, unlike other pain treatments such as opioids.
What are the safety concerns
Common adverse effects of suzetrigine may include itching(2.1%), rash(0.5%), muscle spasms(1.3%), and increased levels of creatine kinase(0.5%). Mild side effects may include nausea, constipation, headache, and dizziness.
Concomitant use of suzetrigine with strong CYP3A inhibitors is contraindicated
This medicine may temporarily reduce the chance of females becoming pregnant while taking this medicine. Talk to your healthcare provider if you have concerns about becoming pregnant. If you are using contraceptives, you should continue to use contraceptives during treatment with Journavx.
What are the alternatives
There are no other substitutes for this first in class drugs. But there are several companies actively working on the me-too or me-better drugs, like Jiangsu Hengrui, Shanghai Huilun Pharma,Wuhan Humanwell Healthcare, and etc.
